Chemistry tutor 10975 views see similar chemistry a level tutors.
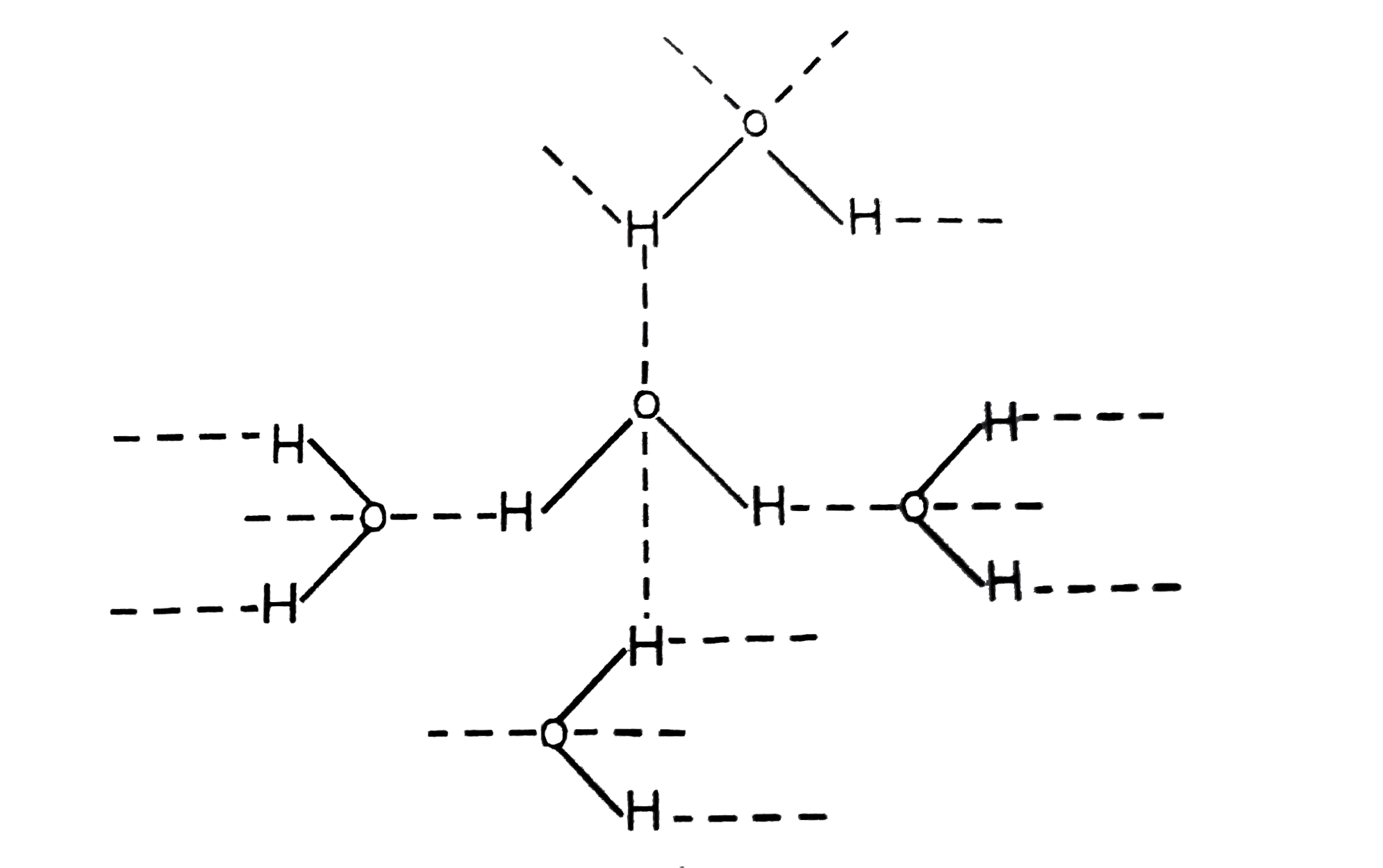
Why water is a liquid at room temperature but hydrogen sulphide is a gas.
Hence water has a higher boiling point or is a liquid at room temperature whereas h 2 s is a gas at room temperature.
Have you registered for the pre jee main pre aipmt 2016.
H2s hydrogen sulfide is a gas because at room temperature the forces and interactions between the molecules of hydrogen sulfide are very weak.
So lesser energy is required to overcome the forces of interaction between the hydrogen sulphide molecules than those between water molecules.
The water molecule has a stronger dipole a.
Although hydrogen sulphide has a greater molecular mass than water water is a liquid at room temperature while carbon dioxide is a gas because of the strong intermolecular forces that hold water.
H2s hydrogen sulfide is a gas because at room temperature the forces and interactions between the molecules of hydrogen sulfide are very weak.
The water molecule has a stronger dipole a negatively and positively charged end to the individual molecule so these negative and positive ends continually attract each other like magnets of opposite poles creating more cohesion between the molecules of water explaining the liquid phase.
This energy is available at room temperature and hence hydrogen sulphide is a gas while water is still a liquid.
Paper by super 30 aakash institute powered by embibe analysis improve your score by 22 minimum while there is still time.
Answered by melissa k.