Because surface waters are in equilibrium with atmospheric carbon dioxide there is a constant concentration of carbonic acid h2co3 in the water.
Why does acid rain affect granite more than limestone.
Acid rain is created by air pollutants.
This is not very soluble so rocks don t dissolve very quickly.
The presence of limestone and other calcium carbonate rock in lakes and streams helps to maintain a constant ph because the minerals react with the excess acid.
One of the most noticeable effects of acid rain is on limestone blocks that are part of a building or statue.
But if you add an acid you add hydrogen ions h which will react with the carbonate to form hydrogen carbonate hco3 ions which are very soluble in water and the limestone will dissolve.
Granite has little or no effect to acid rain whilst limestone is slowly eroded therefore granite lasts longer.
The chemicals fall to earth as acid rain.
Acid precipitation affects stone primarily in two ways.
Stone surface material may be lost all over or only in spots that are more reactive.
Sulphur dioxide a byproduct of industrial development and burning fossil fuels combines with nitrogen oxide an air pollutant created by car exhaust furnaces boilers and engines.
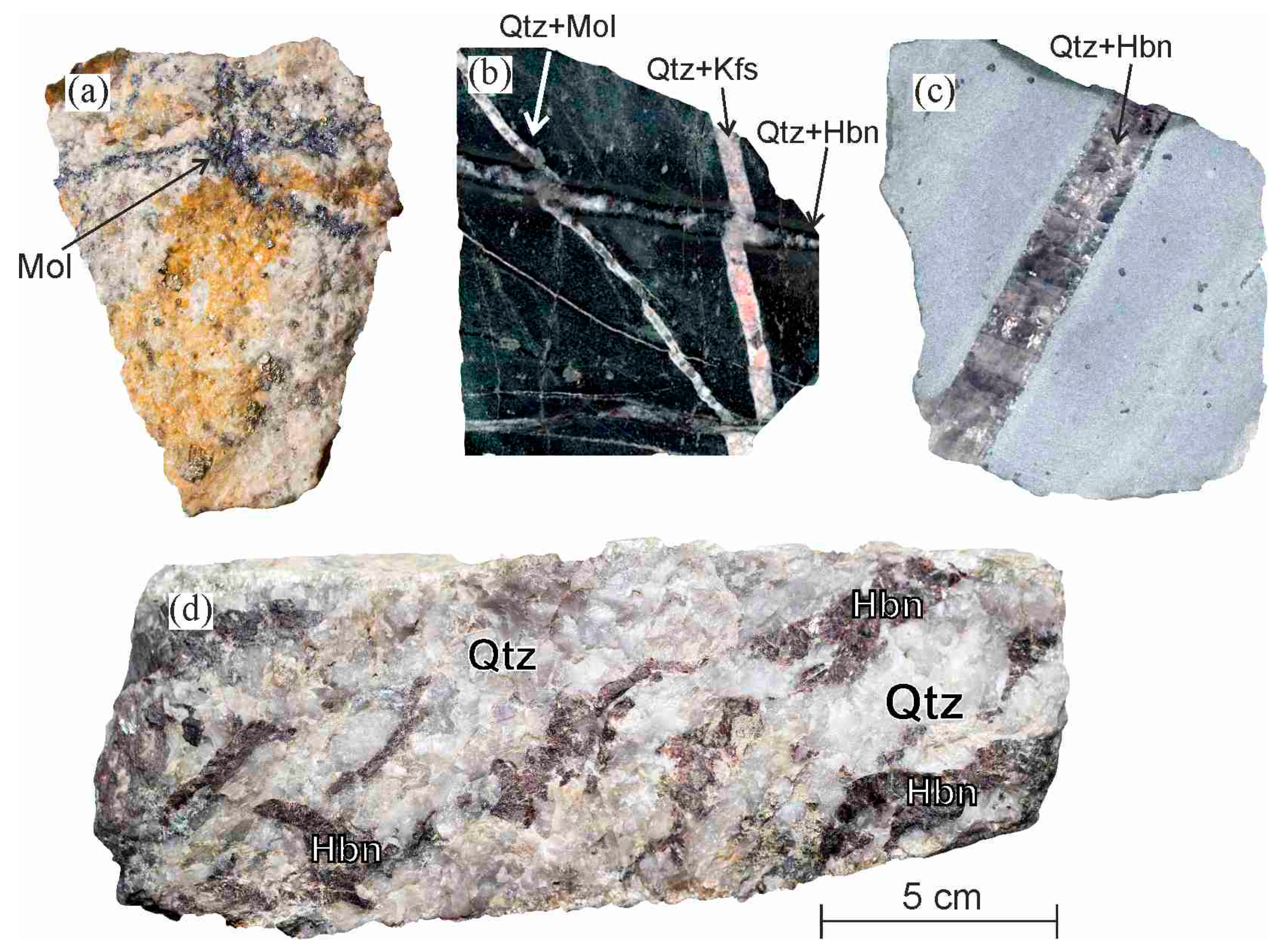
Limestone is mostly made up of the mineral calcium carbonate caco3.
Over time the acid rain will round the edges of statues and pit the flat surfaces of the rock.
How does acid precipitation affect marble and limestone buildings.
When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
Another common reaction is the production of gypsum on the surface of the limestone that comes in contact with sulfuric acid.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
Acid rain is rain that has an excess of protons h present usually as a result of pollutants in the air.
Or if there is more acid two hydrogen ions will.
Limestone is a rock that is composed of calcium carbonate caco3.